Question 1.
You have been provided with three test tubes. One of them contains distilled
water and the other two contain an acidic solution and a basic solution,
respectively. If you are given only red litmus paper, how will you identify
the contents of each test tube?
Answer.
Take a small volume of all three liquids in
three different test tubes. Dip red litmus paper strips separatelyin all the
three test tubes. The tube in which red litmus strip turns blue, contains a
basic solution. Now, we use the blue litmus paper as testing paper and dip it
into the remaining two solutions. The solution which changes the colour of the
blue litmus paper into red is acidic and the other which does not affect it,
is neutral, i.e. distilled water.
Question 2.
Why should curd and other sour substances not be kept in containers made up
of brass or copper?
Answer.
Both curd and other sour substances contain
some acids in them. They react with copper or brass vessels to form certain
salts which are not good for health. Therefore, it is not advisable to keep
them in brass or copper containers.
Question 3.
Which gas is usually liberated when an acid
reacts with a metal? Illustrate with an example. How will you test for the
presence of this gas?
Answer.
Metals are mostly reactive in nature. They
react with dilute acids (HCl and H
2SO
4) to evolve hydrogen gas. For example,
Zn(s) + 2HCl(aq) ➝ ZnCl
2(aq) + H
2(g)
The gas burns with a pop sound when a
burning candle is brought near to it.
Question 4.
A metallic compound A’ reacts with dilute
hydrochloric acid to produce effervescence. The gas evolved extinguishes a
burning candle. Write a balanced chemical equation for the reaction if one of
the compounds formed is calcium chloride.
Answer.
The gas evolved with effervescence and
extinguishes a lit candle is CO
2. If one of the compounds formed is CaCl
2, the reaction would be
CaCO
3(s) + HCl(aq) ➝ CaCl
2(aq) + CO
2 + H
2O(l)
Question 5.
Aqueous solutions of HCl, HNO
3 and H
2SO
4, etc. show acidic character while those of the compounds like ethyl alcohol
(C
2H
5OH) and glucose (C
6H
12O
6) fail to do so. Explain.
Answer.
All the listed acids have replaceable
hydrogen atoms which they release in aqueous solution as hydrogen ions.
Therefore, they show acidic character. However, both ethyl alcohol (C
2H
5OH) and glucose (C
6H
12O
6) do not undergo dissociation in aqueous solution. That’s why they do not
conduct electricity in aqueous solution.
Question 6.
Why does an aqueous solution of an acid
conduct electricity?
Answer.
Aqueous solution of an acid releases H
+ and H
3O
+ in solution. Since ions are carriers of charge, therefore they are
responsible for conducting electricity.
Question 7.
Why does dry HCl gas not change the colour
of dry litmus paper?
Answer.
A dry HCl gas has no H
+ so it does not show any acidic character therefore, no change in colour
takes place until we moisten the litmus paper.
Question 8.
While diluting an acid, why is it
recommended to add acid to water and not water to the acid?
Answer.
Mineral acids such as, H
2SO
4, HNO
3, HCl, etc. have strong affinity for water, so dilution of acid is highly
exothermic in nature. This heat may cause jumping of solution or cracking of
apparatus. In order to avoid it, acid is added drop by drop to water which
dilutes the heat and prevent accident.
Question 9.
How is concentration of hydronium ions (H
3O
+) affected when solution of an acid is diluted?
Answer.
An acid dissociates into hydronium ions (H
3O+) and anions when dissolved in water. Upon dilution, the volume of the
solution increases and the number of ions per unit volume decreases.
Therefore, the concentration of H
3O
+ per unit volume decreases.
Question 10.
How is concentration of hydroxyl (OH
–) ions affected when excess of base is dissolved in solution of sodium
hydroxide?
Answer.
Sodium hydroxide (NaOH) is a strong base.
It immediately dissociates in solution to give OH
– and cations. Upon dissolving more of the base in the solution, the
concentration of OH
– further increases.
Question 11.
You have two solutions A and B. The pH of
solution A is 6 and that of solution B is 8. Which solution has more hydrogen
ion concentration? Which of these is acidic and which one is basic?
Answer.
The pH of a solution is inversely
proportional to the concentration of H
+ in solution. Lesser the pH of the solution, more will be the H
+ concentration. The solution A with pH 6 has more H
+ concentration than the solution with pH equal to 8. The solution A is
acidic because its pH is less than 7 and the solution B is basic because its
pH is more than 7.
Question 12.
What effect does concentration of H
+(aq) have on acidic nature of a solution?
Answer.
The acidic nature of a solution is directly
related to the concentration of H
+. As the concentration of H
+ increases, the acidic nature of the solution also increases.
Question 13.
Do basic solutions also have H
+(aq)? If yes, then why are these basic?
Answer.
Yes, basic solutions also have H
+. As the solutions are prepared in water and water being a weak electrolyte,
it dissociates into H
+ and OH
– but the number of H
+ are very small as compared to OH
– ions.
Question 14.
Under what soil conditions, do you think a
farmer would spread or treat the soil of his field with quick lime (calcium
oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Answer.
A soil usually becomes acidic when there is
either a high peat content or, iron minerals or there are some rotten
vegetables in the soil. In order to reduce the acidic strength, ‘liming of
soil’ is usually done. For this, any of the substances that have been
mentioned are added to the soil since they are of basic nature.
Question 15.
Name the substance which upon treating with
chlorine gives bleaching powder. Write the chemical equation for the reaction.
Answer.
Slaked lime is the substance which reacts
with chlorine to give bleaching powder.
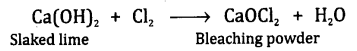
Question 16.
Name the sodium compound used for softening
hard water.
Answer.
Washing soda or sodium carbonate
decahydrate
(Na
2CO
3.10H
2O)
Question 17.
What will happen if the solution of sodium
hydrogen carbonate is heated? Write the chemical equation involved.
Answer.
Carbon dioxide gas will evolve and sodium
car-bonate will be formed.
2NaHCO
3 ➝ Na
2CO
3 + CO
2 + H
2O
Question 18.
Write the chemical equation for the
reaction between Plaster of Paris and water.
Answer.

Question 19.
What is the common name of the compound
CaOCl
2?
Answer.
The common name of the compound CaOCl
2 is bleaching powder.
Chapter End Questions
Question 1.
A solution turns red litmus blue. Its pH is
likely to be
(a) 2
(b) 4
(c) 7
(d) 10
Answer.
(d) 10
Question 2.
A solution reacts with crushed egg-shells
to give a gas that turns lime water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Answer.
(b) HCl
Question 3.
10 mL of solution of NaOH is found to be
completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of
the same solution of NaOH, the amount of HCl solution (the same solution as
before) required to neutralise will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Answer.
(d) 16 mL
Question 4.
Which of the following types of medicines
is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer.
(c) Antacid
Question 5.
Write the word equation and the balanced
equations for the reactions when:
(a) dilute
sulphuric acid reacts with zinc granules.
(b) dilute
hydrochloric acid reacts with magnesium ribbon.
(c) dilute
sulphuric acid reacts with aluminium powder.
(d) dilute
hydrochloric acid reacts with iron filings.
Answer.
(a) Word
equation:
Zinc + Sulphuric add ➝ Zinc
sulphate + Hydrogen
Balanced equation:
Zn(s) + H
2SO
4(dil.) ➝ ZnSO
4(aq) + H
2(g)
(b) Word
equation:
Magnesium + Hydrochloric acid ➝
Magnesium chloride + Hydrogen
Balanced
equation:
Mg(s) + 2HCl (dil.) ➝ MgCl
2(aq) + H
2(g)
(c) Word
equation:
Aluminium + Sulphuric acid ➝
Aluminium sulphate + Hydrogen
Balanced
equation:
2Al(s) + 3H
2SO
4(dil.) ➝ Al
2(SO
4)
3(aq) + 3H
2(g)
(d) Word
equation:
Iron + Hydrochloric acid ➝ Iron
chloride + Hydrogen
Balanced equation:
Fe(s) + 2HCl(dil.) ➝ FeCl
2(aq) + H
2(g)
Question 6.
Compounds such as alcohol and glucose also
contain hydrogen but are not characterised as
acids. Describe an activity to prove it.
Answer.
Compounds such as alcohol and glucose also
contain hydrogen but do not behave like an acid. Both are organic compounds
with the formulae C
2H
5OH and C
6H
12O
6, respectively. This can be proved by the following activity:
In a glass beaker, take a dilute solution
of glucose (C
6H
12O
6). Fix two small nails of iron in a rubber cork and place the cork in the
beaker as shown in the figure. Connect the nails to the terminals of a 6 volt
battery through a bulb. Switch on the current. The bulb will not glow. This
shows that the electric current has not passed through the glucose solution.
As the current is carried by the movement of ions, it shows that the solution
of

glucose has not given any H
+. Now repeat the same experiment with ethyl alcohol (C
2H
5OH). The bulb will again not glow.
This
shows that both of them do not behave as acids although they contain hydrogen
atoms in their molecules.
Question 7.
Why does distilled water not conduct
electricity whereas rain water does?
Answer.
Pure or distilled water has no ions as it
is a very weak electrolyte. So, no conduction of electricity takes place but
rain water contains dissolved acids and so rain water is a good conductor of
electricity.
Question 8.
Why does an acid not show any acidic
behaviour in the absence of water?
Answer.
The acidic behaviour of a substance is due
to the presence of H
+(aq) ions. As acids do not dissociate to produce H
+(aq) ions in the absence of water so they do not show acidic behaviour.
Question 9.
Five solutions A, B, C, D and E when tested
with universal indicator show pH as 4, 1, 11, 7 and 9, respectively. Which
solution is:
(a) neutral
(b) strongly
alkaline
(c) strongly
acidic
(d) weakly
alkaline
(e) weakly
acidic
Arrange the pH in increasing order
of H
+ concentration.
Answer.
(a) Neutral:
D with pH = 7
(b) Strongly
alkaline: C with pH = 11
(c) Strongly
acidic: B with pH = 1
(d) Weakly
alkaline: E with pH = 9
(e) Weakly
acidic: A with pH = 4
Increasing order of H
+ concentration:
C<E<D<A<B
Question 10.
Equal lengths of magnesium ribbons are
taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A
while acetic acid (CH
3COOH) is added to test tube B. In which case, fizzing occurs more vigorously
and why? Fizzing in the reaction is due to the evolution of hydrogen gas by
the action of metal on the acid.
Mg(s) +
2HCl(aq) ➝ MgCl2(aq) + H
2(g)
(A)
Mg(s) + 2CH
3COOH(aq) ➝ (CH
3COO)
2Mg(aq) + H
2(g)
(B)
Since hydrochloric acid is a stronger acid
than acetic acid, fizzing occurs more readily in tube A than in tube B.
Actually hydrogen gas will evolve at more brisk speed in test tube A.
Question 11.
Fresh milk has a pH of 6. How do you think
the pH will change as it turns into curd? Explain your answer.
Answer.
When milk changes into curd, the pH
decreases. Lactose (carbohydrate) present in milk gets converted into lactic
acid. As more acid is formed, pH of the medium decreases.
Question 12.
A milkman adds a very small amount of
baking soda to fresh milk.
(a) Why does
he shift the pH of the fresh milk from 6 to slightly alkaline?
(b) Why does
milk take a long time to set as curd?
Answer.
(a) Fresh
milk is slightly acidic due to the presence of lactic acid. The presence of
bacteria decreases the pH of milk and makes it sour. To prevent it, baking
soda (NaHCO
3) is added to neutralise the acidic nature making it slightly alkaline.
(b) When milk
changes to curd, it becomes more acidic but adding baking soda neutralises it
and checks curdling.
Question 13.
Why should Plaster of Paris be stored in a
moisture-proof container?
Answer.
In the presence of moisture, Plaster of
Paris gets hydrated and changes to gypsum which is a hard mass.

It can no longer be used for making moulds and statues. Therefore, Plaster of Paris is kept in moisture proof containers or bags.
Question 14.
What is neutralisation reaction? Give two
examples.
Answer.
Neutralisation reaction is the reaction
between an acid and a base dissolved in aqueous solution to form salt and
water.
Acid + Base ➝ Salt + Water
HCl(aq) + NaOH(aq) ➝ NaCl(aq) + H
2O(l)
HNO
3(aq) + KOH(aq) ➝ KNO
3(aq) + H
2O(l)
Both NaCl and KNO
3 are neutral in nature. They neither change blue litmus red nor red
litmus blue. That is why the reaction is called neutralisation reaction.
Question 15.
Give two important uses of washing soda and
baking soda.
Answer.
•
Uses of Baking Soda: It is used:
- as an antacid to neutralise excess acidity in the stomach.
- as an ingredient in certain food stuffs such as bread, cake, etc.
- in soda-acid fire extinguishers.
- in making aerated soft drinks.
- to produce carbon dioxide
• Uses of Washing Soda: It is used:
- as a domestic cleaning agent.
- for removing permanent hardness of water.
- as a laboratory reagent.
- in the manufacture of sodium compounds such as borax.
- in the manufacture of glass, paper and soap.